Current state of evidence
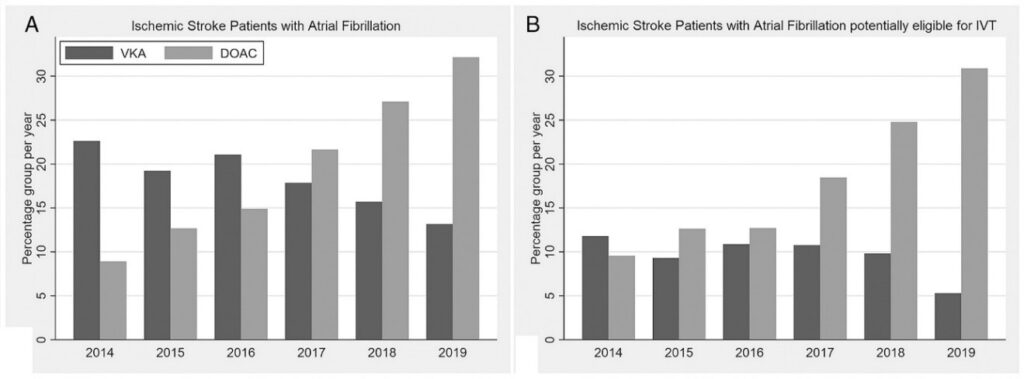
(A) Percentages of patients with atrial fibrillation among all ischemic stroke patients. (B) Percentages of patients with atrial fibrillation within the subgroup of ischemic stroke patients potentially eligible for intravenous thrombolysis. Note the decrease in VKA pretreatment with concomitant rapid increase in DOAC pretreatment in both patient groups. DOACs = direct oral anticoagulants; IVT = intravenous thrombolysis; VKA = vitamin K antagonist.
In the National Swiss Stroke Registry, we assessed the frequency of prior anticoagulation with DOACs, and the utilization of IVT in consecutive patients (2014–2019, Figure 1). We found that among 8’179 Atrial Fibrillation patients, 1,634 (20%) were on DOAC treatment at stroke onset with increasing rates in recent years and even higher rates among those potentially eligible for IVT. 1–7
In a nationwide analysis covering the years 2015-2019, we found that among patients potentially eligible for IVT, the rate of IVT was 74% (95% CI 72–76%) in controls, 63% (57–69%) in patients on VKA (with international normalized ratio ≤1.7), and only 15% (12–18%) in patients on DOACs.8 This reflects the uncertainty felt by clinicians about whether to apply IVT in this clinical situation despite the fact that most stroke units and centers followed a dedicated standard operating procedure to guide treatment decisions over the study timeframe. Despite previous efforts, the rate of IVT was more than five times lower in patients on DOACs than in controls.
On the basis of this data, we established a multi-center, global, retrospective, observational cohort study to address the unanswered question whether recent DOAC intake is associated with hemorrhagic complications after IVT.9 This study included 64 primary and comprehensive stroke centers across Europe, Asia and Australia/New Zealand. The exposure was DOAC therapy (confirmed last ingestion <48 hours prior to IVT) as compared to no oral anticoagulation. The overall result was that recent use of DOACs (<48 h) was not associated with increased risk of sICH regardless whether patients were selected based on DOAC plasma levels, reversal with idarucizumab or neither.
Alteplase Background
Alteplase is known as recombinant tissue plasminogen activator (rtPA). 10% of the dose is given as a bolus with the 90% remaining given as a continuous infusion over 60 minutes. The efficacy of alteplase is time-sensitive, particularly in ischemic stroke, where prompt intervention is crucial. Extensive clinical trials have demonstrated the efficacy of alteplase in improving patient outcomes when administered, especially within the early therapeutic window (up to 4.5 hours after onset).10
Despite its benefits with regards to functional outcome, alteplase is associated with potential risks, including bleeding complications. Careful patient selection and adherence to established protocols are essential to mitigate these risks.11
Tenecteplase Background
Tenecteplase (TNK) is a fibrin-specific plasminogen activator thrombolytic agent showing promising results in acute ischemic stroke treatment. TNK has advantageous pharmacological characteristics and ease of administration when compared to tPA i.e. bolus administration alone, instead of bolus followed by a continuous infusion.12 Specifically, TNK has higher fibrin specificity, increased resistance to plasminogen activator, and longer half-life. Several randomized-controlled clinical trials have also evaluated the use of intravenous TNK in patients presenting with an acute ischemic stroke.13,14
According to the recent 2019 ESO/ESMINT guidelines on MT, seven out of eleven experts recommended TNK over alteplase if the vessel occlusion status is known at the time point of decision-making.15 As of now, no prospective evaluation of administering intra-arterial TNK in patients with incomplete reperfusion is available.
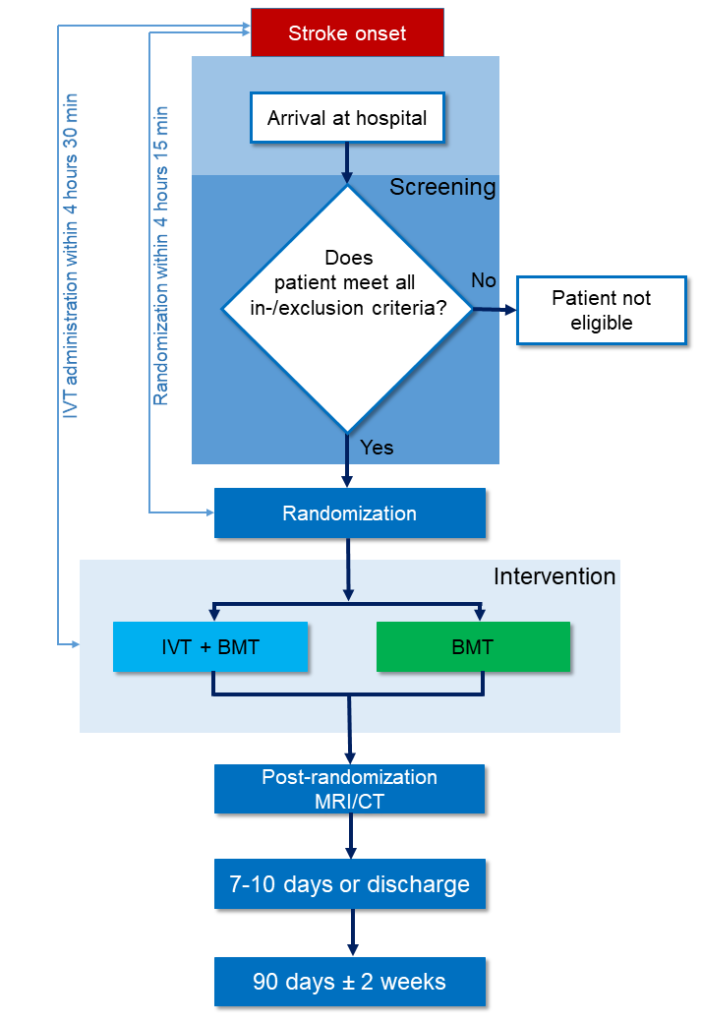
Trial design
DO-IT is a multicenter, prospective, randomized, open label, blinded endpoint (PROBE) trial with predefined interim analyses. The experimental arm is defined as administration of IVT plus BMT. The standard arm is defined as BMT with no additional study-specific treatment (Figure 2). 906 patients will be randomly assigned to either the standard or the experimental arm with a ratio of 1:1 (453 patients per group).
Randomization
The trial is designed as a randomized (1:1) parallel group controlled trial. Allocation of patients will be done once the patient fulfills all inclusion and exclusion criteria. Patients will be randomly assigned in a ratio of 1:1 to one of two treatment arms:
- IVT (intravenous administration of Tenecteplase/Alteplase) + BMT
- Standard of care/BMT according to local applicable guidelines, including the current American Heart Association/American Stroke Association (AHA/ASA)7 and European Stroke Organisation/ European Society of Minimally Invasive Neurological Therapy (ESO/ESMINT) guidelines.5,16,17
Outcomes
The primary efficacy outcome is the mRS at 90 days ± 2 weeks after admission.
Key secondary efficacy outcomes are 1) Dichtomized good functional outcome (mRS 0-2 or return to baseline at day 90 ± 2 weeks), 2) Change in stroke severity (National Institutes of Health Stroke Scale and ischemic stroke volume) between baseline and 24 ± 12 hours, 3) Health-related Quality of Life (EuroQol 5D-3L) at 90 days ± 2 weeks.
Key secondary safety outcomes are 1) symptomatic intracranial hemorrhage within the first 36 hours (European Co-operative Acute Stroke Study-II definition), 2) major extracranial bleeding within the first 24 hours, and 3) all-cause mortality up to 7 days after admission or until discharge.
Key inclusion and exclusion criteria
Key inclusion criteria
- Informed consent (deferred consent when possible according to national legislation)
- AIS eligible to receive intravenous alteplase/tenecteplase as per standard of care disabling according to the judgement of the treating physician
- DOAC ingestion within 48 hours prior to enrollment, or patient with an ongoing prescription of DOAC but exact time point of last intake is unknown.
- Either
- Can be randomized within 4 hours 15 minutes and treated within 4 hours 30 minutes of last known well time OR
- MRI showing a pattern of “DWI-FLAIR-mismatch”, i.e. acute ischemic lesion visibly on DWI (“positive DWI”) but no marked parenchymal hyperintensity visible on FLAIR (“negative FLAIR”) indicative of an acute ischemic lesion ≤4.5 hours of age AND Treatment can be started within 4.5 hours of symptom recognition (e.g., awakening)
Key exclusion criteria
- Contra-indications to IVT by the current standard of care of the treating physicians with the exception of recent DOAC intake as specified above.
- Intended reversal by specific or unspecific reversal agents
- Pregnancy or lactating women. To be reasonable sure to exclude women with ongoing pregnancy, women are not considered of childbearing potential if they fulfill the following criteria
- Age > 55 years OR
- Age < 55 years and at least 12 months since last menstrual period OR
- Have had a documented surgical sterilization
- Patient < 18 years of age (since the benefit of IVT is unproven in this population)
Since the benefit of IVT might be smaller in patients in which additional endovascular treatment is planned59, we will cap patients with intended mechanical thrombectomy at 20% of the trial population. If this number is reached, the following additional exclusion criterion will be applied:
- Intended treatment with endovascular reperfusion strategies
Trial structure
Steering Committee
- Prof. Dr. Diana Aguiar de Sousa
- Prof. Dr. Bruce Campbell
- Prof. Dr. Luciana Catanese
- Dr. Jonathan Coutinho
- Prof. Dr. Gian Marco De Marchis
- Prof. Dr. Jesse Dawson
- Prof. Dr. Urs Fischer
- Prof. Dr. Dr. Johannes Kaesmacher
- Assoc. Prof. Dr. Aristeidis Katsanos
- Prof. Dr. Masatoshi Koga
- Prof. Dr. Robin Lemmens
- PD Dr. Thomas Meinel
- Prof. Dr. Carlos Molina
- Prof. Dr. Jan Purrucker
- Prof. Dr. David Seiffge
- Prof. Dr. Mike Sharma
- Prof. Dr. Daniel Strbian
- Assoc. Prof. Dr. Ashkan Shoamanesh
- Prof. Dr. Luciano Sposato
- Prof. Dr. Götz Thomalla
- Prof. Dr. Sven Trelle
- Prof. Dr. Georgios Tsivgoulis
- Prof. Dr. Guillaume Turc
- Prof. Dr. David Werring
- Dr. Teddy Wu
Data Safety Monitoring Board
Prof. Dr. Prof. Rustam Al-Shahi Salman
Prof. Dr. Charlotte Cordonnier
Dr. Nikki Rommers
Literature
- World Medical Association Declaration of Helsinki. JAMA 2013; 310: 2191.
- International Council on Harmonization (ICH, 1996) E6 Guideline for Good Clinical Practice.
- International Council on Harmonization (ICH, 1997) E8 Guideline: General Considerations for Clinical Trials.
- European Parliament and the Council of the European Union. Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. Off J Eur Union 2014; 1–76.
- Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet 2022; 400: 161–169.
- Alamowitch S, Turc G, Palaiodimou L, et al. European Stroke Organisation ( ESO ) expedited recommendation on tenecteplase for acute ischaemic stroke. 2023; 47–83.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke A. Stroke 2019; 50: E344–E418.
- Meinel TR, Branca M, De Marchis GM, et al. Prior Anticoagulation in Patients with Ischemic Stroke and Atrial Fibrillation. Ann Neurol 2021; 89: 42–53.
- Meinel TR, Wilson D, Gensicke H, et al. Intravenous Thrombolysis in Patients With Ischemic Stroke and Recent Ingestion of Direct Oral Anticoagulants. JAMA Neurol; online ahe. Epub ahead of print January 2023. DOI: 10.1001/jamaneurol.2022.4782.
- Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935.
- European Medical Agency. Actilyse.
- Giannandrea D, Caponi C, Mengoni A, et al. Intravenous thrombolysis in stroke after dabigatran reversal with idarucizumab: Case series and systematic review. J Neurol Neurosurg Psychiatry 2019; 90: 619–623.
- Šaňák D, Jakubíček S, Černík D, et al. Intravenous Thrombolysis in Patients with Acute Ischemic Stroke after a Reversal of Dabigatran Anticoagulation with Idarucizumab: A Real-World Clinical Experience. J Stroke Cerebrovasc Dis 2018; 27: 2479–2483.
- Fang CW, Tsai Y Te, Chou PC, et al. Intravenous Thrombolysis in Acute Ischemic Stroke After Idarucizumab Reversal of Dabigatran Effect: Analysis of the Cases From Taiwan. J Stroke Cerebrovasc Dis 2019; 28: 815–820.
- Ploen R, Sun L, Zhou W, et al. Rivaroxaban does not increase hemorrhage after thrombolysis in experimental ischemic stroke. J Cereb Blood Flow Metab 2014; 34: 495–501.
- Beharry J, Waters MJ, Drew R, et al. Dabigatran Reversal Before Intravenous Tenecteplase in Acute Ischemic Stroke. Stroke 2020; 10–13.
- Chen HS, Cui Y, Zhou ZH, et al. Effect of Argatroban Plus Intravenous Alteplase vs Intravenous Alteplase Alone on Neurologic Function in Patients with Acute Ischemic Stroke: The ARAIS Randomized Clinical Trial. Jama 2023; 329: 640–650.
